Science
Targeting the GABAA receptor in peripheral tissues is a novel drug mechanism of action
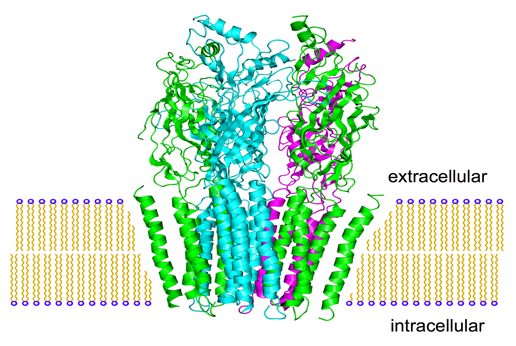
GABAA Receptors are well characterized ion channels
Gamma aminobutyric acid (GABA) subtype A receptors (GABAAR) are integral membrane ion channels comprised of five individual protein subunits, most often associated with inhibitory neurotransmission in the central nervous system. Nineteen discrete GABAAR subunits (α1–6, β1–3, γ1–3, δ, ε, θ, π, and ρ1–3) have been identified, which can assemble into pentameric channels that can have unique anatomic locations, functional characteristics, and responses to pharmacological agents. GABAAR chloride ion channel activity is modulated by endogenous GABA and allosterically with pharmacological ligands. GABAARs are validated drug targets for human drugs in use for over 50 years.
- Peripheral GABAAR modulation
- GABAAR are known CNS targets of anesthetic, anxiolytic, antiseizuere and sedative drugs.
- Recent discoveries show simolar GABAAR structure/function outside the CNS
- Multiple GABAAR subunits enable design of tissue selective drugs
- Pantherics’ peripherally acting agents are designed to avoid CNS exposure.
- Targeted beneficial biological effects in the periphery
- Smooth muscle relaxation
- Anti-inflammatory
During the last two decades, a growing body of research has identified GABAAR subunits and their functional ion channels in many non-neuronal cells, including a restricted subset of GABAAR on smooth muscle and inflammatory cells (T lymphocytes, splenocytes, mast cells, macrophages, monocytes, and polymorphonuclear cells). Peripheral anatomical localizations include lung, bladder, intestinal track, skin, heart, and kidney. Although targeting GABAAR in the brain is a widely employed and effective therapeutic strategy, this validated mechanism of action has not been exploited for peripheral (non-centrally acting) disease indications. Pantherics is the first company to systematically apply knowledge of GABAAR structure, function, and ligand chemistry through the design and development of a safe, non-centrally acting, orally available new drug class, with a focus on inflammatory disorders of smooth muscle. The company scientists and collaborative network are on the forefront of selective GABAAR ligand design and corresponding drug development.
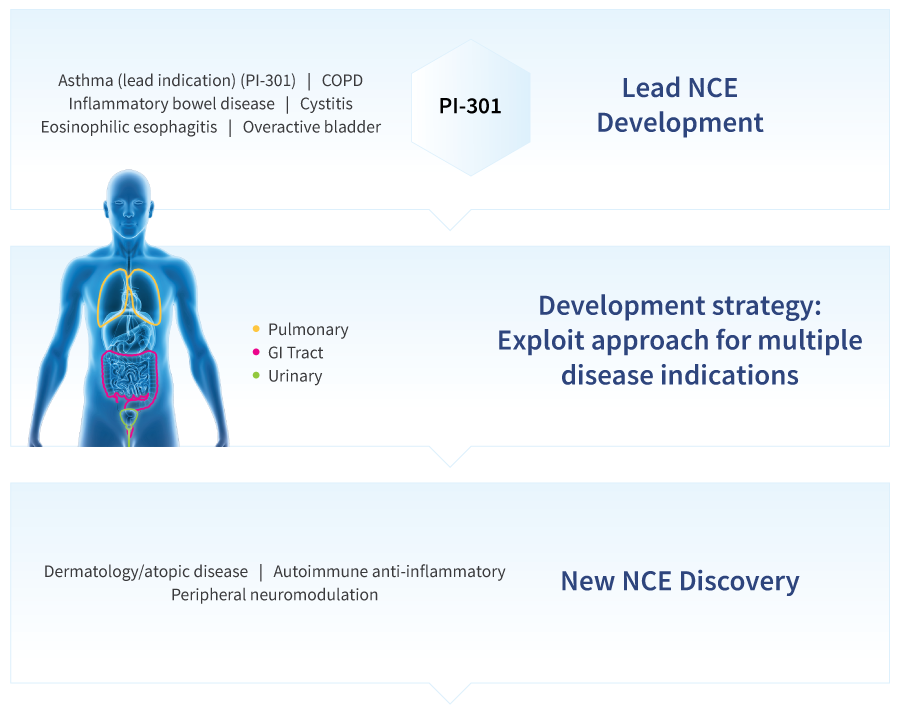
The lead indication from Pantherics’ core technology is novel drug for symptom control in persistent asthma and its acute exacerbations, where GABAAR targeting is a fundamentally novel therapeutic mechanism of action. This non-steroidal, non-GPCR approach is also being developed for symptom control in chronic obstructive pulmonary disease (COPD) and other lung disorders. The research and development pipeline includes novel drug compositions for digestive, urinary, and dermatological indications.
Pantherics is the first company to systematically develop drugs based on peripheral GABAA Receptor targeting
Inflammatory disease of smooth muscle:
opportunities in respiratory disease
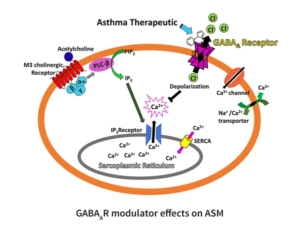
Chronic inflammatory disorders of the lung can alter airway smooth muscle (ASM) structure and function, resulting in impaired breathing and acute bronchospasm. Our drug development approach targets GABAAR in the lung to decrease inflammation and improve ASM function, with an initial focus on asthma.
ASM constrict upon interaction with acetylcholine that binds the M3 cholineric receptor. This G-protein coupled receptor upregulates phosphoinositide phospholipase C, which in turn catalyzes the hydrolysis of phosphatidylinositol (PIP2) to inositol triphosphate (IP3) and diacylglycerol (DAG). IP3 binds to the IP3 receptor located on the surface of the sarcoplasmic reticulum (SR) and calcium (Ca2+) is released into the cytosol 1. Following the release, an influx of Ca2+ via the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) occurs to refill the SR Ca2+store, a process referred to as store-operated calcium entry (SOCE)2.
We have shown that GABAAR modulators reduced the increase of intercellular Ca2+ upon extracellular activation, resulting in airway smooth muscle relaxation and reduction of calcium oscillations in ex vivo airway smooth muscle (Figure 2) 3. The resting membrane potential of ASM is thought to be approximately -60 mV and the reversal potential of chloride is approximately -20 to -30 mV 4, thus binding of allosteric modulators to the GABAAR is expected to depolarize the cell influencing the function of membrane proteins such as SERCA, voltage sensitive Ca2+ channels, and reverse Na+/Ca2+ exchangers.
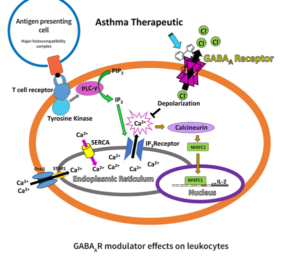
Leukocytes such as eosinophils, T lymphocytes, and macrophages play an important role in asthma. Large numbers of eosinophils are normally present in asthmatic airways to protect against pathogens and environmental irritants, however, their activation, overproduction of cytotoxic proteins (degranulation), and release of reactive oxygen species maintain chronic asthma 5. Asthma is considered to be driven by a TH2 cell inflammatory process with notable involvement of interleukins 4, 5, and 13 secreted by CD4+ T lymphocytes, among other pro-inflammatory mediators (Figure 3) 6. IgE class switching, mast cell recruitment, eosinophilia, mucus production, and airway hyperresponsiveness contribute to disease pathology.
Antigen-driven T cell activation signaling pathways lead to a significant transport of Ca2+ from the endoplasmic reticulum (ER) to the cytoplasm, which in turn activates serine/threonine protein phosphatase calcineurin leading to dephosphorylation and activation of NFAT proteins that migrate to the nucleus to induce the transcription of interleukins 7. The production of interleukins mediates chronic inflammation, a hallmark of asthma (Figure 4).
The ER Ca2+ store is replenished via the endoplasmic reticulum Ca2+ ATPase (SERCA) and Ca2+ release-activated Ca2+ channels (CRAC) such as Orai1 in conjunction with Stim1 8. We have shown that allosteric GABAAR modulators reduced the increase of intercellular Ca2+ of stimulated T cells and reduced the secretion of IL-2 9. Electrophysiological studies showed that allosteric GABAAR modulators directly increase the transmembrane current in a dose depending manner 10 .