Clinical Lead Product
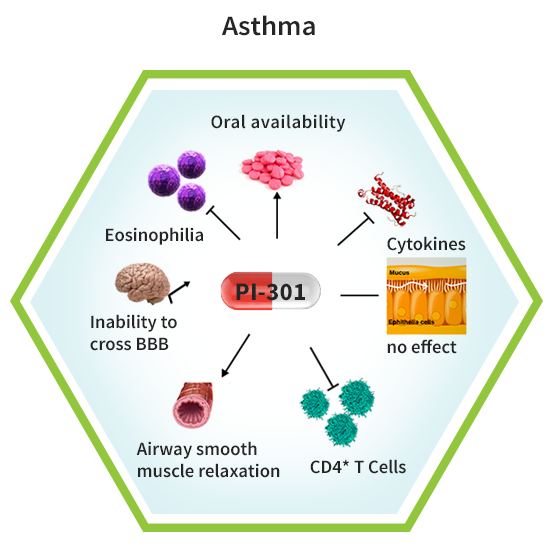
Summary of pharmacological effects of PI-301
Oral availability:
PI-301 (MIDD0301) is a small molecule, GABAAR agonist formulated for oral administration 1 and is therefore expected to improve treatment compliance over common inhaled asthma controller medications. Over 50% of asthma patients use their inhalers improperly; reducing dosing efficacy and increasing the risk of oral candidiasis (thrush) 2. Furthermore, β2 adrenergic receptor agonists used in asthma treatment are non-tissue selective and when administered orally can cause hypertension and slow heart rate that can lead to heart failure. Multiple in vivo studies in animal asthma models have shown that PI-301 is effective with once a day dosing, demonstrating protection against airway hyperresponsiveness and reducing lung inflammation 3. Usually, once a day oral medication supports compliance of 80% and higher. A recently published scale-up synthesis of PI-301 enables cost-effective manufacturing (CMC) 4.
Airway smooth muscle relaxation:
Airway smooth muscle cells are present in the upper airways and can increase in mass in asthma, contributing to airway remodeling. PI-301, and several members of this compound class, interact directly with GABAAR on these cells causing relaxation of pre-constricted human airway smooth muscle cells 5,6,7,8.
Recently, our group showed that nebulized PI-301 rapidly relaxes airway smooth muscle in mice after 10 seconds of inhalation 9. Furthermore, studies using ex vivo human airway smooth muscle pre-constricted with histamine exhibited muscle relaxation following treatment with PI-301.
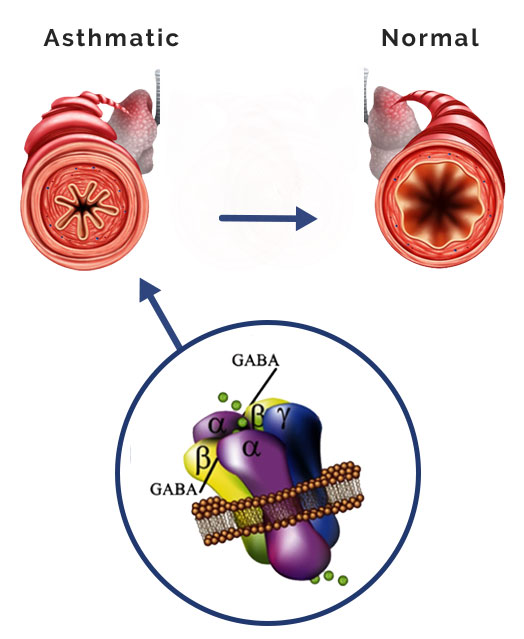
PI-301’s effects on asthmatic bronchial passages
Lack of CNS effects:
Inhaled corticosteroids are well distributed and reduce lung inflammation, but also suppress hypothalamic–pituitary–adrenal axis activity, associated with growth suppression, glaucoma, cataracts, shin thinning and bruising, bone fractures, and osteoporosis 10. In addition, corticosteroids have central nervous system (CNS) effects and cause, among others, depression, cognitive deficits, insomnia, and attention deficits 11. PI-301 was designed to have limited CNS distribution and associated CNS adverse effects. We have shown that several compounds, including PI-301, have excellent lung distribution following oral dosing but without significant brain exposure 3.
Reduction of eosinophil numbers:
Large numbers of eosinophils are present in the asthmatic airways as a result of inflammatory signaling. Resulting overproduction of cytotoxic proteins (degranulation) and excretion of reactive oxygen species, as well as activation of macrophages contribute to the pathophysiology of chronic asthma 12. Reduction of eosinophil numbers by inhibiting their differentiation from progenitor cells and recruitment to the lung mediated by interleukin-5 (IL-5), among other agents, has led to the therapeutic use of IL-5 antibodies in asthma. We have shown that small molecules such as PI-301 can reduce eosinophil numbers as well, presenting a more cost-effective and orally available option for the first-line treatment of asthma 3.
Mucus hypersecretion:
A hallmark of asthma is mucus cell metaplasia caused by the differentiation of epithelial cells induced by TH2 lymphocyte cytokines IL-4, IL-9, and IL-13 13. The hypersecretion of mucus leads to airway obstruction and is mediated by the upregulation of the MUC5AC genes that produces a gel forming glycoprotein. The reduction of mucus in the lung is still a pharmaceutical challenge and even inhibition of IL-4 with soluble IL-4 receptor protein did not result in an efficacious drug 14. PI-301 did not alter mucus metaplasia in mice, however, current efforts are directed in this research area 3.
Reduction of T cells and secreted cytokines:
Asthma is considered to be a TH2 cell driven inflammatory disease sustained by CD4+ T cells and their production of cytokines, especially interleukin IL-4, IL-5, and IL-13 15. We have shown that CD4+ T cell numbers are significantly reduced by PI-301, even at dosages lower than those required to reduce eosinophil numbers 3. Consequently, lung cytokine expression of IL-17A, IL-4, and TNF-α were reduced by PI-301 in a mouse model of asthma without changing anti-inflammatory cytokine IL-10 levels. In addition, we showed that the transmembrane current of CD4+ T cells is modulated in the presence of PI-301, demonstrating that changes of chloride homeostasis reduce activation of CD4+ T cells and attenuate inflammation.
No observable toxicity:
International regulatory bodies require that investigational new drugs be evaluated for immunosuppressive potential (e.g., FDA Guidance, Oct. 2002 and ICH Expert Working Group, European Union, Japan and US). These studies are especially important for investigational drugs that are designed to reduce inflammation. A 28-day repeat dose immunotoxicity evaluation of PI-301 (100 mg/kg, b.i.d.) did not reveal signs of general toxicity as determined by animal weight, organ weight, or hematology 16. In contrast, 5 mg/kg prednisone-treated mice gained less weight over the course of the study and exhibited reduced spleen and thymus weights. Unlike, prednisone, PI-301 did not change circulating lymphocyte, monocyte, and granulocyte numbers and did not alter IgG antibody responses to DNP following DNP-KLH immunization, indicating that systemic humoral immune function was not affected 16.
References: