Pantherics just published a new study investigating the effect of nebulized PI-301 in moderate and severe murine asthma models. The work has been published in ACS Pharmacology and Translation Science titled: Nebulized MIDD0301 Reduces Airway Hyperresponsiveness in Moderate and Severe Murine Asthma Models.
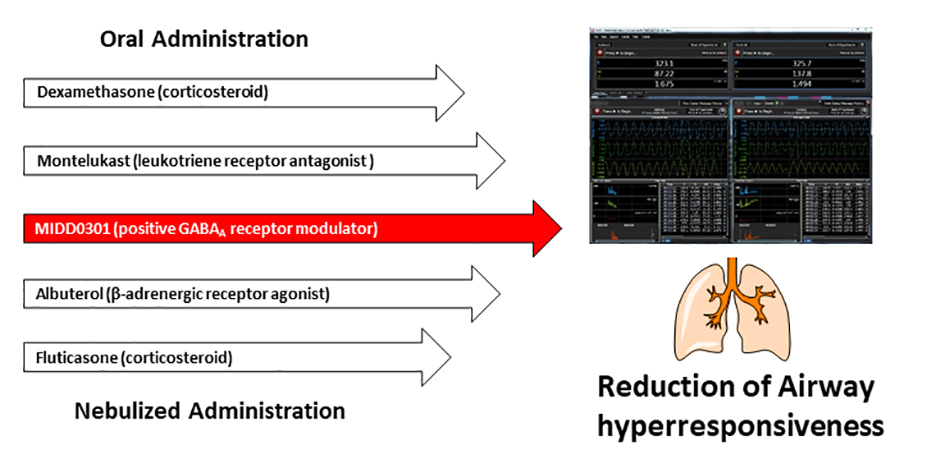
We report the relaxation of methacholine-constricted airways with nebulized PI-301, a positive allosteric γ-aminobutyric acid type A receptor (GABAAR) modulator. The therapeutic efficacy of nebulized PI-301 in reducing airway resistance was investigated in spontaneous breathing mice using a whole-body plethysmograph and in unconscious mice using a forced oscillation technique. Prophylactic nebulized PI-301 reduced subsequent methacholine-induced bronchoconstriction in ovalbumin and house dust mite allergic asthma models and in normal mice. Nebulized PI-301 exhibited comparable or better therapeutic potency compared to nebulized albuterol and oral montelukast. Prophylactic nebulized PI-301 was also effective in reducing bronchoconstriction, comparable to nebulized albuterol or fluticasone, in a steroid resistant asthma mouse model induced by intratracheal installation of lipopolysaccharide and interferon-gamma. Oral dexamethasone was ineffective in this model. Nebulized PI-301 was also effective in reversing bronchospasm when dosed after methacholine challenge comparable to albuterol. Pharmacokinetic studies showed that about 0.06% of nebulized PI-301 entered the mouse lung when using a whole body plethysmograph and therapeutic levels were sustained in the lung for at least 25 min. Consistent with previous reports on orally dosed PI-301, high doses of nebulized PI-301 resulted in minimal brain exposure and thus no observable adverse sensorimotor or respiratory depression effects occurred. In addition, no adverse cardiovascular effects were observed following 100 mg/kg i.p. dosing. These results further demonstrate that charged imidazodiazepine PI-301 can selectively target lung GABAAR without adverse motor, cardiovascular, or respiratory effects and inhaled dosing is effective in reducing bronchoconstriction in allergen and infectious lung inflammation.